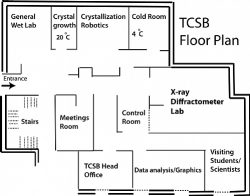
Location & Floor Layout
Built on the 1st floor of the Emerson Family Building for Life Sciences, the TCSB consists of a general biochemical lab for sample preparation; a temperature-controlled crystal growth room; a crystallization room; a cold room at 4 °C; spacious diffractometer and control rooms; a molecular graphics & data analysis room with Mac computing stations; a meetings room, and another office space for up to additional 5 students and/or visiting scientists. The TCSB state-of-the-art systems for macromolecular crystallography are outlined below.
ÄKTA avant
As part of our effort to establish a protein production and purification unit under the TCSB, we are happy to announce we are providing new ÄKTA Avant chromatography system services.
Read more here: https://lokeycenter.net.technion.ac.il/protein-purification-by-chromatography/
Mosquito

The crystallization experiments start by mixing the protein sample with the many crystallization solutions. In the past such extensive screenings required large quantities of pure protein samples at high concentrations, which was a limiting step for many projects with challenging protein purification procedures. Today there are several crystallization robots on the market that reduce the amount of protein needed for a screen dramatically by dispensing volume in the nanolitter range. At the TCSB we use one of the best robots, MOSQUITO, to reproducibly mix the protein with the many solutions for crystallization via either the hanging- or the sitting-drop vapor diffusion method.
Rock Imager

Once mixed with protein the crystallization plates are sealed and incubated at temperature-controlled environments for a few days to a several months to allow slow and controlled crystal growth. The crystallization plates need to be monitored regularly under microscope to identify crystals or lead conditions in which crystal nuclei appear. To do that efficiently in a high-throughput manner we use an automated imaging system (ROCK IMAGER). This robust, easy-to-use robot, incubates and captures high quality images of up to a 1000 micro-plates on a user-defined schedule.
FORMULATOR
 After screening the initial crystallization conditions, the FORMULATOR robot is frequently used for obtaining an optimization screen, based on successful conditions.
After screening the initial crystallization conditions, the FORMULATOR robot is frequently used for obtaining an optimization screen, based on successful conditions.
This robot can mix up to 16 different solutions, each at a desired volume, to generate various compositions of the individual solutions.
The resultant formulations are dispensed onto a desired plate format, suitable for the crystallization experiments.
Rock Maker Software
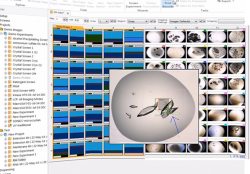

Rock Maker is a powerful, yet ,easy-to-use, software that manages the entire protein crystallization process. This software controls and enables crystallization experiment design, automated image scheduling for the Rock Imager and a simple layout for examining and scoring the crystallization drops.
The Rock Maker software also allows the design of an optimized screen for the Formulator robot to dispense.
Manual imaging and crystal manipulations is conveniently carried out using our Ziess Discovery V20 stereo microscope, with motorized 20x zoom. The microscope is hooked up to a high quality digital camera for computerized imaging, and to a UV light source for discriminating protein from salt crystals.
X-ray Diffractometer
 Once crystals of suitable size (above 20 microns) are obtained they are tested for their X-ray diffraction quality. Use of X-ray beam of higher intensity increases the resolution of the collectable data, and this is where the quality of the X-ray source comes into play. The recent advances in brightness of home laboratory sources of X-rays, now allow us to become almost independent of expensive synchrotron time and travels, and collect data in house for most of the biological questions we investigate. The TCSB houses the highest intensity rotating-anode X-ray generator on the market, FR-X by Rigaku, with beam brightness comparable to 2ndgeneration synchrotrons.
Once crystals of suitable size (above 20 microns) are obtained they are tested for their X-ray diffraction quality. Use of X-ray beam of higher intensity increases the resolution of the collectable data, and this is where the quality of the X-ray source comes into play. The recent advances in brightness of home laboratory sources of X-rays, now allow us to become almost independent of expensive synchrotron time and travels, and collect data in house for most of the biological questions we investigate. The TCSB houses the highest intensity rotating-anode X-ray generator on the market, FR-X by Rigaku, with beam brightness comparable to 2ndgeneration synchrotrons.
In addition to the traditional Cu anode producing radiation wavelength of 1.56 Å, this is unique generator has a Cu/Cr dual anode technology which to produce radiation at 2.29 Å as well. The Cr radiation enhances anomalous signals from sulfur atoms, which can be used for experimental phasing of native proteins containing Cys or Met residues. This ability could be very useful for structure determination of novel protein structures.
At the TCSB we measure the diffracted X-rays on an image-plate based detector, R-Axis HTC (Rigaku), which combines three large image plates with minimal dead time, good sensitivity in a wide dynamic range, making it ideal for collecting fast and accurate data.
Automated Crystal Mounting System

Collection of a complete data set from a single crystal can take up to few hours or in unique certain circumstances requiring high redundancy a whole day or two may be needed. To take full advantage of our intense beam, an automated system for routine screening of crystals for X-ray diffraction quality and data collection was installed at the TCSB. The ACTOR robot allows mounting screening of up to 80 frozen crystals in a completely automated manner. This technology allows for systematic search for the best crystals or to optimize conditions for data collection, and thus shorten the crystallization to structure determination pipeline significantly.
Data analysis and computation

X-ray reflection data measured on the detector need to be converted to values of intensities, a process that requires significant amount of software processing that is carried out conveniently in our Graphics room. Such computation and solving for the phase of each reflection are done efficiently on our iMac workstations, which in addition offer great graphic capabilities for molecular building and visualization.
Biochemical characterization of biomolecules interaction
TCSB hosts two modern instruments for analyzing molecular interactions and determination of binding affinity (Kd).
 The first one is a highly sensitive Isothermal Titration Calorimeter (NANO ITC) which uses solid state thermoelectric heating and cooling systems to precisely control temperature, and has an injection syringe assembly for efficient and accurate delivery of titrant.
The first one is a highly sensitive Isothermal Titration Calorimeter (NANO ITC) which uses solid state thermoelectric heating and cooling systems to precisely control temperature, and has an injection syringe assembly for efficient and accurate delivery of titrant.
This instrument is designed to measure the enthalpy or the heat released by spontaneously interacting molecules (ΔH). Fitting the binding curves allow determination of the binding constant (Kd), and thus the free energy of interaction (ΔG = RTlnKd) and the entropy (ΔS) is derived from the Gibbs equation: ΔG = ΔH–TΔS.
 The second instrument, the Microscale Thermophoresis (MST), makes use of mobility of molecules along temperature gradients. Upon binding of a ligand, molecules change size, shape, charge and/or their hydration shell and as a result their mobility under an induced temperature gradient.
The second instrument, the Microscale Thermophoresis (MST), makes use of mobility of molecules along temperature gradients. Upon binding of a ligand, molecules change size, shape, charge and/or their hydration shell and as a result their mobility under an induced temperature gradient.
Fluorescent labeling of these molecules enable monitoring mobility changes of very small quantities of the molecule, which can be plotted against varying concentration of ligands for deriving the binding constant Kd. Although no enthalpy or entropy values can be obtained, the advantage of this method lies in its capacity to use small amounts of molecules, which do not need to be pure since only the labeled molecules are being followed.
 Differential Scanning Fluorimetry technology (nanoDSF) is a label-free approach used for measuring protein stability. The technique is based on monitoring changes in the fluorescence of the intrinsic tryptophan and tyrosine amino acids, upon heating of the sample. Stability screening can be performed for antibodies, enzymes and membrane proteins. Aggregation can also be detected by using a backreflection technology. Protein solutions can be analyzed independent of buffer compositions, with a maximal protein concentration ranging from 250 mg/ml down to 5 µg/ml. As of now, our facility uniquely provides experiments with the nanoDSF in Israel.
Differential Scanning Fluorimetry technology (nanoDSF) is a label-free approach used for measuring protein stability. The technique is based on monitoring changes in the fluorescence of the intrinsic tryptophan and tyrosine amino acids, upon heating of the sample. Stability screening can be performed for antibodies, enzymes and membrane proteins. Aggregation can also be detected by using a backreflection technology. Protein solutions can be analyzed independent of buffer compositions, with a maximal protein concentration ranging from 250 mg/ml down to 5 µg/ml. As of now, our facility uniquely provides experiments with the nanoDSF in Israel.